10 minutes maximum! Can you do it in 5? |
|||||||||||||||||
Q1+2. When a nucleus emits an α or β particle, it changes to a new element. 1. In this ß decay equation, what are the missing numbers x and y? |
|||||||||||||||||
|
|||||||||||||||||
2. In the above equation, a particle is missing from the right-hand side of the equation, having no mass or charge. What is the name of this particle?
|
|||||||||||||||||
|
3. The half life of an isotope is found to be approximately 1000 years. In a sample, only one eighth (12.5%) of the sample remains, the rest having decayed to a new element.
How old is the sample?
|
|||||||||||||||||
4. There are 4 fundamental forces at work in nature. Which of these answers describes the range of gravity and the strong nuclear force?
| |||||||||||||||||
5. Nuclear power stations emit a small amount of radiation into the environment, which is detectable all the time. this is called background radiation. There are many other sources. |

|
||||||||||||||||
Which of the following is NOT a source of background nuclear radiation?
|
|||||||||||||||||
6. Electrons orbiting the nucleus of an atom have been found to have different levels of energy that are not continuous and have specific values depending on the element. What word in physics is used to describe these 'non-continuous' energy levels?
|
|||||||||||||||||
Q7-10. This diagram shows electron energy levels in a hydrogen atom. Only the first 4 energy levels are shown. Emission or absorption of a photon is associated with an electron jump between energy levels. For the following questions, only jumps between the 4 energy levels shown are considered. |
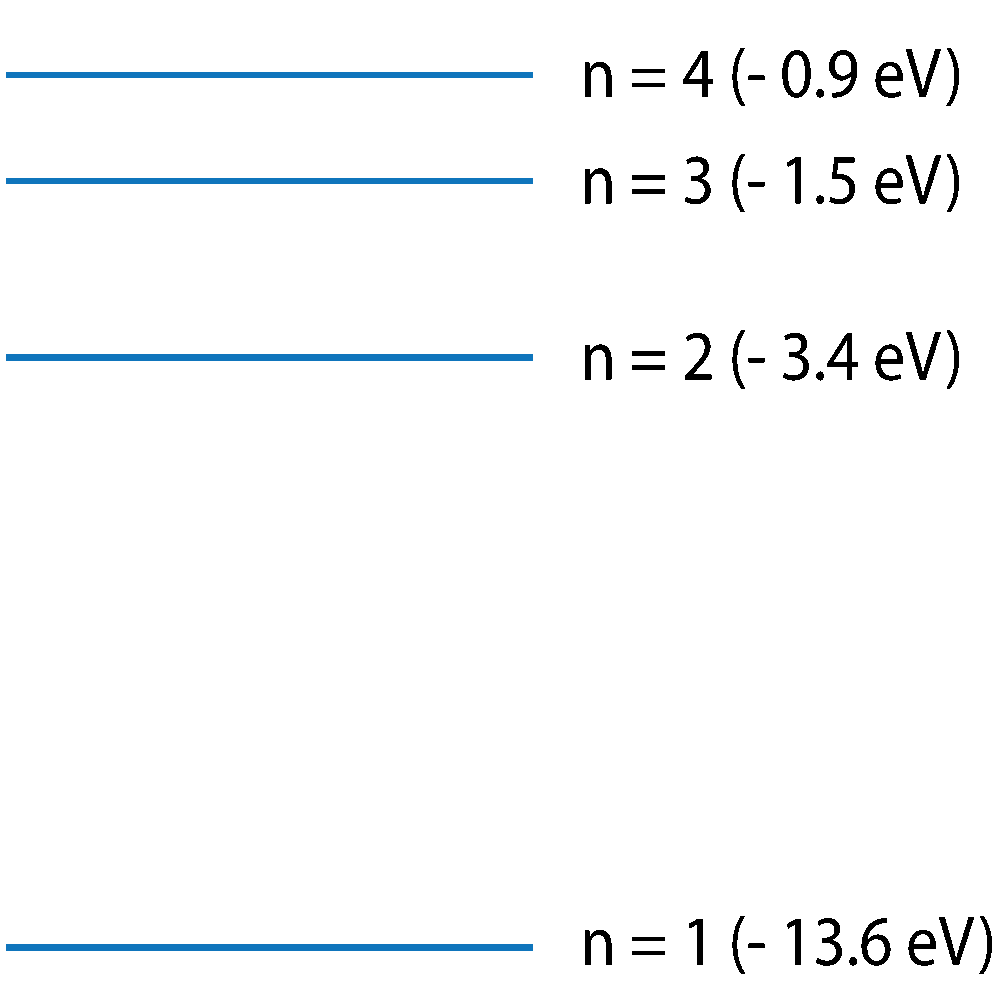
(Not to scale -approximate energy level values). |
||||||||||||||||
7. Which energy level change will emit a photon with the highest frequency?
| |||||||||||||||||
8. At n = ∞, which of these statements is true?
| |||||||||||||||||
9. Cold hydrogen gas in space is subjected to a wide spectrum of radiation from the Sun. Which of these statements best describes the absorption and emission of radiation by the hydrogen gas?
| |||||||||||||||||
10. Photons of visible light have energy of between 1.8 and 3.1 eVs. Which of the following transitions is likely to emit an infrared light photon?
| |||||||||||||||||