NEED HELP? |
  |
How many?
|
||||||||||||||||||||||||||||||||||||||||||||||||||
2+3. Look at this diagram of an atom of a common element.
|
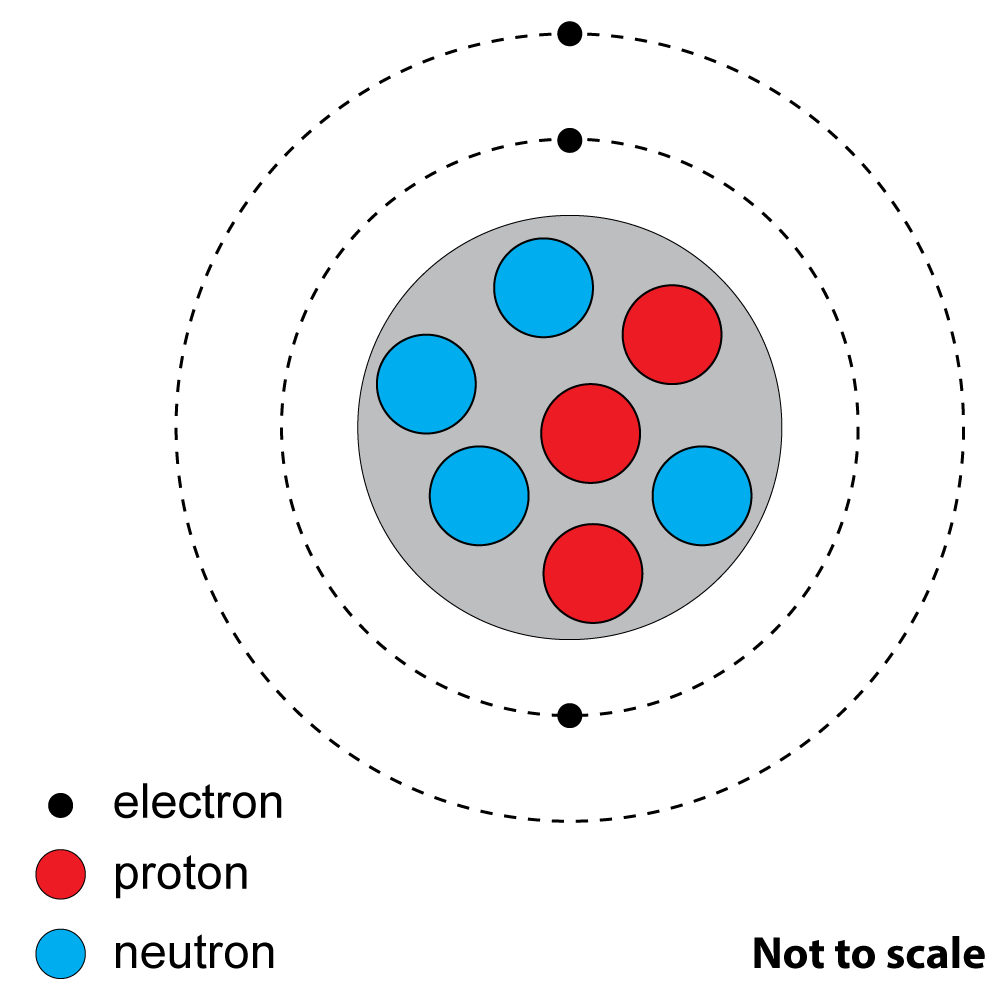 |
|||||||||||||||||||||||||||||||||||||||||||||||||
2. What is the atomic (proton) number? A). 3 |
||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. What is the mass (nucleon) number?
A). 3 |
||||||||||||||||||||||||||||||||||||||||||||||||||
4. Two isotopes of the same element have ... A). the same number of protons but different numbers of neutrons. |
||||||||||||||||||||||||||||||||||||||||||||||||||
5. A radioactive isotope is a substance that ... A). will eventually gain electrons through bonding. |
||||||||||||||||||||||||||||||||||||||||||||||||||
6. Which of the following best describes the words 'contamination' and 'irradiation'?
|
||||||||||||||||||||||||||||||||||||||||||||||||||
Questions 7-9 are about types of radiation. Which type(s) of radiation match the following descriptions? |
 |
|||||||||||||||||||||||||||||||||||||||||||||||||
| 7. The most ionising. | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8. A fast moving electron. | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 9. Can pass through paper. | ||||||||||||||||||||||||||||||||||||||||||||||||||
10. When Uranium decays it emits an alpha particle, forming an isotope of Thorium. Which of the following decay equations is correct?
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| 11. What change takes place to an atom during beta decay?
A). A proton changes into a neutron. |
||||||||||||||||||||||||||||||||||||||||||||||||||
12. In this decay equation, what are the missing numbers x and y?
|
||||||||||||||||||||||||||||||||||||||||||||||||||