|
10 minutes maximum
An IB Periodic Table is required. |
1. Which of the following statements about homologous series is false?
- A. A homologous series is a group of compounds with the same molecular formula.
- B. The members of the homologous series have the same chemical properties.
- C. The compounds of the homologous series share the same functional group.
- D. Each member of the homologous series differs from the next by a common structural unit.
| |
2. What is the IUPAC name for the compound shown in this diagram? |
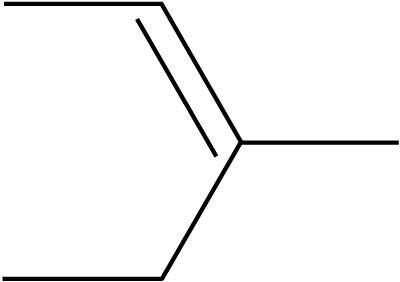 |
- A. 2-ethylbut-2-ene
- B. 3-ethylbut-2-ene
- C. 3-methylpent-2-ene
- D. 3-methylpent-3-ene
|
|
| 3. How many structural isomers are there for C4H8?
| |
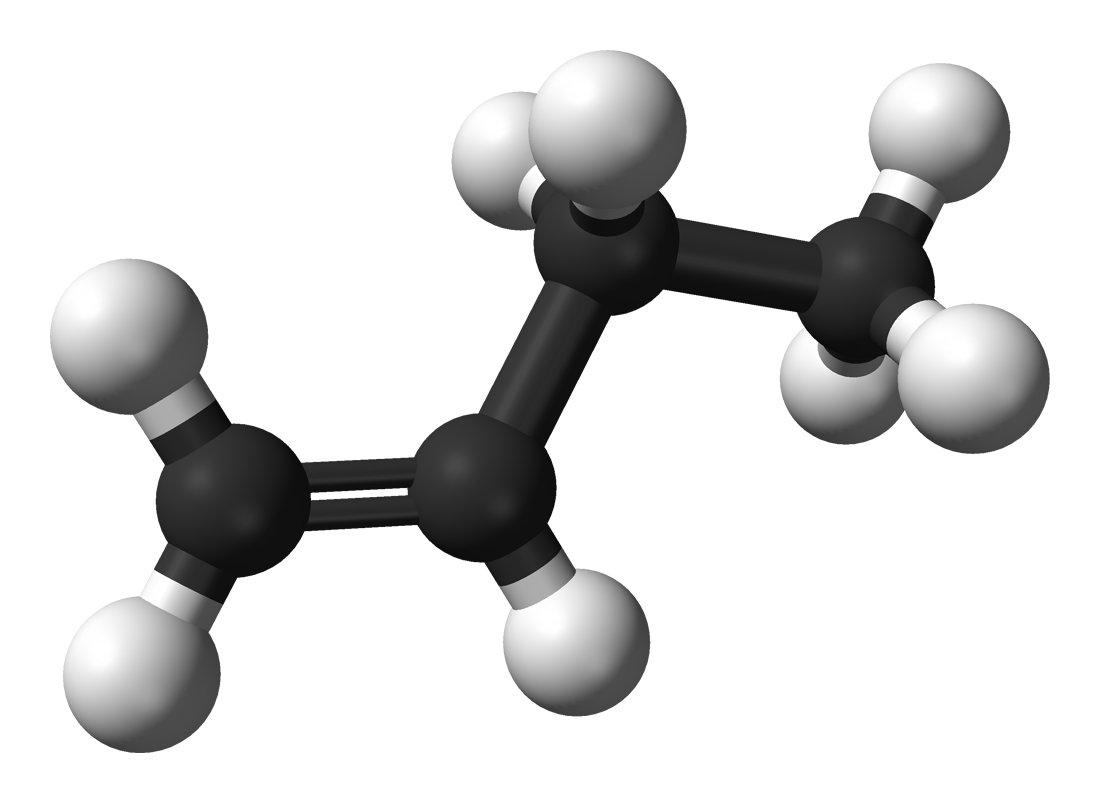
Questions 4-6 are about the six organic compounds below labelled I, II, III, IV, V, VI:
| I. |
II. |
III. |
| Pentan-2-ol |
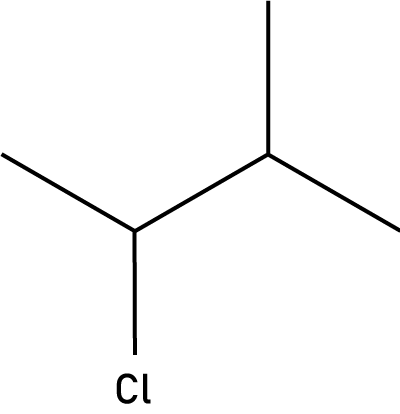 |
CH3CHBrCH3 |
| IV. |
V. |
VI. |
 |
 |
-pent-2-ene.svg) |
|
|
4. Which compounds are members of the halogenoalkane homologous series?
- A. I and V
- B. IV and VI
- C. II and III
- D. I and III
| |
5. Which compounds are unsaturated?
- A. I, II, III
- B. III, IV, VI
- C. I, V, VI
- D. IV, V, VI
| |
6. Which compound is a secondary alcohol?
| |
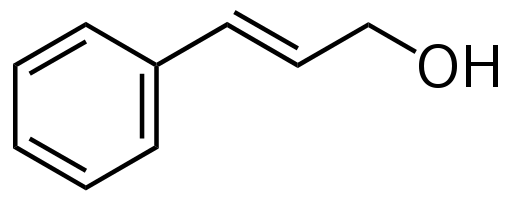
7. Which statements are correct about the molecule below?
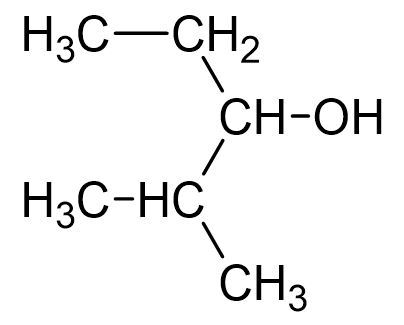
I. Its molecular formula is C6H14O.
II. The molecule has an alcohol functional group.
III. It is a structural isomer of 2-propoxypropane. |
|
|
- A. I only
- B. I and III only
- C. II and III only
- D. I, II and III
| |
8. The molar masses of CH3(CH2)2CH3, CH3(CH2)2NH2 and CH3CH2Cl are similar. What is the correct order of their boiling points?
- A. CH3(CH2)2CH3 < CH3(CH2)2NH2 < CH3CH2Cl
- B. CH3CH2Cl < CH3(CH2)2NH2 < CH3(CH2)2CH3
- C. CH3CH2Cl < CH3(CH2)2CH3 < CH3(CH2)2NH2
- D. CH3(CH2)2CH3 < CH3CH2Cl < CH3(CH2)2NH2
| |
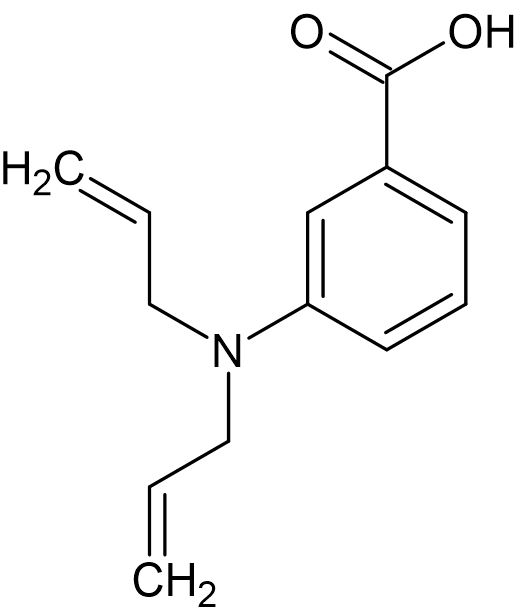
9. Which functional groups are present in this molecule?
|
|
- A. Alkenyl, phenyl, carboxyl, amine
- B. Carbonyl, hydroxyl, alkenyl, phenyl, amine
- C. Carboxamide, alkenyl, hydroxyl, phenyl
- D. Phenyl, alkenyl, carboxyl, nitrile
| |
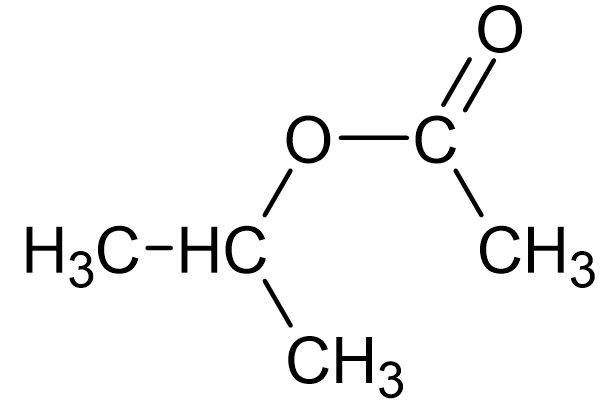
10. What is the IUPAC name of this compound?
|
|
- A. propyl ethanoate
- B. 1-methylethyl ethanoate
- C. ethyl propanoate
- D. ethyl 1-methylethanoate
|
|
|
