|
10 minutes maximum! Can you do it in 5? |
||||||||||||||||||
1. Which of these sentences best describes the specific heat capacity (c) of a substance?
|
||||||||||||||||||
|
2. Which of the following correctly gives the formula for S.H.C. in terms of the heat energy ΔQ supplied to a substance?
|
||||||||||||||||||
3+4. The image shows a large glass vase holding 2 kg of cold water. Water has a specific heat capacity (c) of 4200 J / Kg 0C. |
 |
|||||||||||||||||
3. The energy needed to heat 2 kg of water by 5 0C is ..
| ||||||||||||||||||
3. If I supply 210 kJ of heat to the same 2 kg of water, how much would the temperature increase?
| ||||||||||||||||||
Q 5-7. These questions are about heating different bars of copper. Copper has a specific heat capacity of 800 J / Kg 0C. What are the missing values? |
 |
|||||||||||||||||
|
||||||||||||||||||
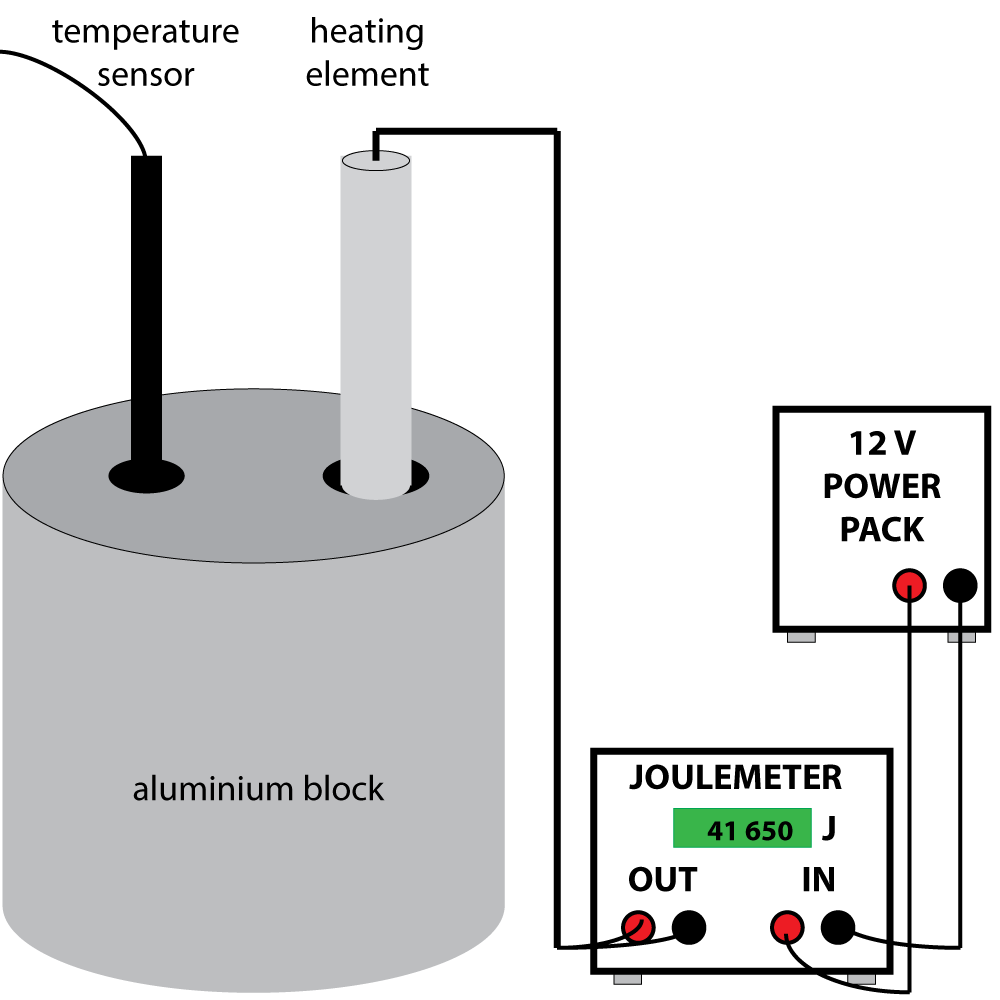
8. The diagram shows a standard method of finding the S.H.C. of aluminium. The block has a mass of exactly 1 kg. What additional measurements are required to find the S.H.C. of aluminium?
|
 |
|||||||||||||||||
|
||||||||||||||||||
| ||||||||||||||||||
9+10. These ice cubes have a total mass of 100g. The specific heat capacity of ice is about half that of water. 9. A student heats these ice cubes and also 200 g of water by 10 oC each. They then compare how much heat energy is needed to do this. Which of these statements is correct? |

Howard wang86 wikimedia commons |
|||||||||||||||||
| 10. In this example, the formula for specific heat capacity can be used when the ice is...
| |||||||||||||||||